CARBON AND ITS COMPOUNDS
When a carbon compound is burnt, CO2 & water are produced. The presence of CO2 can be confirmed by passing it through lime water which turns milky.
All living structures and many non-living structures such as food, clothes, medicines, books etc. are carbon-based.
Earth’s crust has only 0.02% carbon (as minerals like carbonates, hydrogen carbonates, petroleum, coal etc.). The atmosphere has 0.03% CO2. But carbon has immense importance.
BONDING IN CARBON – THE COVALENT BOND
Carbon compounds are poor conductors of electricity. So the bonding does not form ions. They have low melting & boiling points as compared to ionic compounds.
Carbon Compounds | Melting point (K) | Boiling point (K) |
Acetic acid (CH3COOH) | 290 | 391 |
Chloroform (CHCl3) | 209 | 334 |
Ethanol (CH3CH2OH) | 156 | 351 |
Methane (CH4) | 90 | 111 |
Atomic number (Z) of Carbon= 6.
Electronic configuration= 2, 4 (1s2 2s2 2p2).
Carbon has 4 electrons in the outermost shell. Gaining or losing 4 electrons is not possible to attain noble gas configuration because:
- Gaining 4 electrons (C4– anion) makes it difficult to hold 6 protons and 10 electrons.
- Losing 4 electrons (C4+ cation) needs high energy to leave 6 protons and two electrons.
The simplest molecule formed by the sharing of valence electrons is that of hydrogen (Z= 1). It has one electron in K shell and needs one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a hydrogen molecule (H2) and attain the nearest noble gas (helium- 2 electrons in K shell) configuration.
To represent valence electrons, dots or crosses are used.
The shared pair of electrons constitute a covalent bond between 2 hydrogen atoms. It is represented by a line.
Electron dot structure of Chlorine:
Atomic number = 17.Electronic configuration= 2, 8, 7 (7 electrons in valence shell). It forms a diatomic molecule (Cl2).
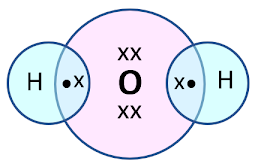
Electron dot structure of Oxygen:
It requires two more electrons to complete its octet.
So the oxygen atom shares 2 electrons with another oxygen atom forming a double bond.
Electron dot structure for water (H2O):
Electron dot structure of Nitrogen:
It forms a diatomic molecule (N2).
To attain an octet, each nitrogen atom in a nitrogen molecule contributes 3 electrons forming triple bond.
Electron dot structure for methane (CH4):
Methane is one of the simplest compounds of carbon.It is used as a fuel and is a major component of biogas and Compressed Natural Gas (CNG).
Valency of Hydrogen= 1.
Valency of Carbon= 4.
Carbon shares these electrons with 4 hydrogen atoms to get a noble gas configuration.
The bonds formed by sharing of an electron pair between two atoms are called covalent bonds.
Covalently bonded molecules have strong bonds within the molecule, but intermolecular forces are weak. This gives rise to the low melting & boiling points.
Since the electrons are shared between atoms and no charged particles are formed, covalent compounds are generally poor conductors of electricity.
ALLOTROPES (DIFFERENT FORMS) OF CARBON
E.g. Diamond, Graphite & Fullerenes.In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One bond is double-bond to satisfy valency. Hexagonal arrays are placed in layers one above the other.
Diamond is the hardest substance. Graphite is smooth and slippery and a very good conductor of electricity.
Synthetic diamonds can be produced by subjecting pure carbon to very high pressure and temperature. These are small but indistinguishable from natural diamonds.
CARBON AND ITS COMPOUNDS
VERSATILE NATURE OF CARBON
This outnumbers the compounds formed by all the other elements put together.
1. CATENATION
It is the ability of carbon to form bonds with other atoms of carbon, giving rise to large molecules.
They may be long chains, branched chains or ring forms.
2. TETRAVALENCY
Carbon compounds are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine etc. giving specific properties.
Carbon atom is small-sized. So the nucleus can hold the shared pairs of electrons strongly. So carbon can make very stable compounds with other elements. The bonds formed by elements having bigger atoms are weaker.
It was thought that organic or carbon compounds could only be formed with the help of a vital force (i.e., a living system is needed).
Friedrich Wöhler (1828) disproved this by preparing urea from ammonium cyanate.
But carbon compounds, except for carbides, oxides of carbon, carbonate and hydrogencarbonate salts are studied under organic chemistry.
Saturated and Unsaturated Carbon Compounds
Chains of carbon atoms contain more carbon atoms. E.g.
No. of C | Name | Formula | Structure |
1 | Methane | CH4 | |
2 | Ethane | C2H6 | |
3 | Propane | C3H8 | |
4 | Butane | C4H10 | |
5 | Pentane | C5H12 | |
6 | Hexane | C6H14 |
Carbon ‘skeleton’ of 4 carbon atoms has two forms:
Complete molecules for two structures with formula C4H10
Will you be my Friend?
Carbon also bonds with other elements such as halogens, oxygen, nitrogen & sulphur.
Some functional groups in carbon compounds
Homologous Series
Homologous series for alkanes: Succeeding members differ by a –CH2- unit. E.g.
CH4 and C2H6 – differ by a –CH2- unit
C2H6 and C3H8 – differ by a –CH2- unit
C3H8 and C4H10 – differ by a –CH2- unit
General formula for alkenes is CnH2n [n = 2, 3, 4].
General formula for alkanes is CnH2n+2.
General formula for alkynes is CnH2n-2.
As the molecular mass increases, physical properties such as melting & boiling points, solubility in solvent etc. also increase. But chemical properties remain similar.
Homologous series of Alcohols:
Compounds | Difference in formula | Difference in molecular mass |
CH3OH & C2H5OH | –CH2- | 14 U |
C2H5OH & C3H7OH | –CH2- | 14 U |
C3H7OH & C4H9OH | –CH2- | 14 U |
C4H9OH & C5H11OH | –CH2- | 14 U |
Nomenclature of Carbon Compounds
Method of naming a carbon compound:
1. Identify the number of carbon atoms. E.g. three-carbon compound is named propane.
2. Presence of functional group is indicated by a prefix or a suffix.
3. If the suffix of the functional group begins with a vowel, the final letter ‘e’ is deleted from the name of the carbon chain. E.g., Propane with a ketone group is named as
Propane – ‘e’ = propan + ‘one’ = propanone.
4. For unsaturated carbon chain, the final ‘ane’ is substituted by ‘ene’ or ‘yne’. E.g., propene (double bond), propyne (triple bond) etc.
Nomenclature of organic compounds:
CARBON AND ITS COMPOUNDS
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
Combustion
C + O2 → CO2 + heat & light
CH4 + 2O2 → CO2 + 2H2O + heat & light
CH3CH2OH + 3O2 → 2CO2 + 3H2O + heat & light
Why do substances burn with or without a flame?
A flame is produced only when gaseous substances burn. So a candle or LPG burns with a flame.
Wood, coal or charcoal burn with a flame at first due to the volatile substances in them. After that they just glow red and gives out heat.
Atoms of gas substance are heated and glow to produce flame. Each element produces characteristic colour. E.g. heating a copper wire in flame gives bluish green flame.
Yellow colour of a candle flame is due to the incomplete combustion of carbon particles. When light falls on them, they scatter yellow colour.
Formation of coal and petroleum (fossil fuels)
Fossil fuels were formed from biomass by biological and geological processes.
Millions of years ago, trees, ferns and other plants were crushed into the earth due to earthquakes or volcanic eruptions. They were pressed down by layers of earth and rock. They slowly decayed into coal.
Dead marine tiny plants and animals sank to the sea bed and were covered by silt. Due to bacterial action, they turned into oil & gas under high pressure. The silt was compressed into rock. The oil & gas seeped into porous rock parts, and got trapped like water in a sponge.
Oxidation
Addition Reaction
Substitution Reaction
Saturated hydrocarbons are unreactive and inert in the presence of most reagents.
However, in the presence of sunlight, hydrocarbons undergo a substitution reaction very fast. E.g.
CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)
Here, chlorine replaces the hydrogen atoms one by one.
Higher homologues of alkanes can form many products.
CARBON AND ITS COMPOUNDS
SOME IMPORTANT CARBON COMPOUNDS: ETHANOL & ETHANOIC ACID
Properties of Ethanol
Reactions of Ethanol
a. Reaction with sodium:
Alcohols react with sodium evolving hydrogen. E.g. Drop a small piece of sodium into pure ethanol. It produces sodium ethoxide (2CH3CH2O–Na+) & H2.
2Na + 2CH3CH2OH → 2CH3CH2O–Na+ + H2
b. Reaction to give unsaturated hydrocarbon:
Heating ethanol at 443 K with excess conc. H2SO4 results in dehydration of ethanol to give ethene. Conc. H2SO4 is a dehydrating agent (removes water from ethanol).
Properties of Ethanoic acid (Acetic acid)
Reactions of ethanoic acid:
b. Reaction with a base:
Ethanoic acid reacts with a base like NaOH to give a salt (sodium ethanoate or sodium acetate) and water.
NaOH + CH3COOH → CH3COONa + H2O
c. Reaction with carbonates & hydrogen carbonates:
2CH3COOH + Na2CO3 → 2CH3COONa + CO2 + H2O
(Sodium acetate)
Pass the gas produced through lime-water. Lime-water turns milky. It means that the gas is CO2.
Reaction with sodium hydrogen carbonate:
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
CARBON AND ITS COMPOUNDS
SOAPS AND DETERGENTS
Take 10 mL of water each in two test tubes A & B.
At the surface of water, soap aligns such that its ionic end is in water and hydrocarbon tail protrude out of water.
Inside water, these molecules form clusters in which the hydrophobic tails are oriented towards interior and the ionic ends towards exterior. This cluster is called a micelle.
The oily dirt is collected in the centre of micelle. The micelles stay as a colloid and will not come together to precipitate because of ion-ion repulsion. So, the dirt in micelles is easily rinsed away.
The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
The water containing sulphates/ chlorides/ hydrogen carbonates of calcium or magnesium is called hard water. E.g. Water from tube well or hand-pump.
- Take 10 mL distilled water (or rain water) and 10 mL hard water in separate test tubes (hard water can be prepared by dissolving salts of Ca or Mg in water).
- Add few drops of soap solution to both and shake well for same period.
- Test tube with distilled water gets more foam.
- Test tube with hard water gets white curdy precipitate.
Detergents
- Take two test tubes with 10 mL hard water in each.
- Add five drops of soap solution to one and five drops of detergent solution to the other.
- Shake both test tubes for the same period.
- The test tube with detergent gets more foam.
- In test tube with soap, curdy precipitate is formed.